All About Allergan Botox
Wiki Article
6 Simple Techniques For Allergan Botox
Table of ContentsWhat Does Allergan Botox Do?Everything about Allergan BotoxAllergan Botox Fundamentals ExplainedNot known Factual Statements About Allergan Botox How Allergan Botox can Save You Time, Stress, and Money.Excitement About Allergan Botox
2013 Apr; 53( 4 ):644655. [Bar, Medication: 23458496] 14. Botox onabotulinumtoxin, A for injection Ph. Eur. Clostridium botulinum type A neurotoxin facility (900k, D); clean and sterile vacuum-dried concentrate powder for solution for injection: 50, 100 and also 200 Allergan devices per vial [item essay] Markham (ON): Allergan, Inc; Dec 21, 2011. 15. Aurora SK, Dodick DW, Turkel CC, De, Gryse RE, Silberstein SD, Lipton RB, et al.Cephalalgia. 2010 Jul; 30( 7):793803. [Club, Med: 20647170] 16. Diener HC, Dodick DW, Aurora SK, Turkel CC, De, Gryse RE, Lipton RB, et al. Onabotulinumtoxin, A for therapy of persistent migraine headache: results from the double-blind, randomized, placebo-controlled stage of the PREEMPT 2 test. Cephalalgia. 2010 Jul; 30 (7):804814. [Bar, Med: 20647171] 17. Clinical research report 191622-079.
Professional research study record 191622-080. Personal inner manufacturer's record]
Ottawa: Wellness Products and Food Branch, Wellness Canada; Aug 17, 2010. Botox injection (onabotulinumtoxin, A) for treatment of frustrations in adults with chronic migraine. Company: Allergan, Inc.
The Single Strategy To Use For Allergan Botox
Application no. Rockville( MD ): Center for Examination and Research Study; Oct 15, 2010. [cited 2014 Jan 8] Statistical testimonial(s) [Net] Offered from: http://www. accessdata. fda.gov/ drugsatfda_docs/ bla/2010/103000Orig1s5215. pdf. 21. Center for Medicine Evaluation and also Research. Botox shot (onabotulinumtoxin, A) for treatment of frustrations in adults with persistent migraine. Firm: Allergan, Inc. Application no. BLA 103000 S-5215 [FDA authorization plan]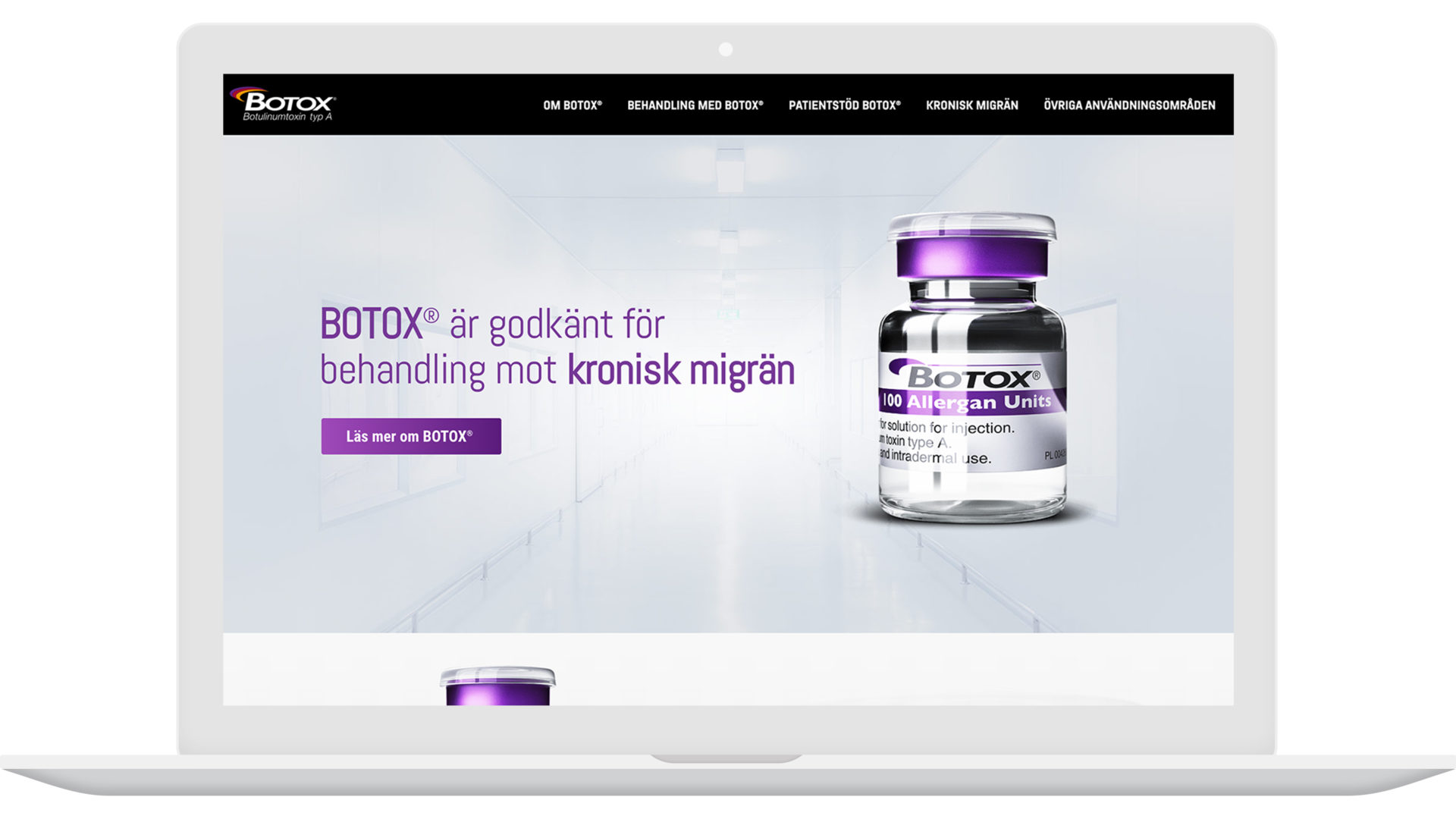
A multi-center double-blind pilot comparison of onabotulinumtoxin, An and topiramate for the prophylactic treatment of chronic migraine headache. Headache. Botulinum toxin kind An and divalproex sodium for prophylactic therapy of episodic or chronic migraine headache.
2008 Feb; 48( 2 ):210220. [Club, Medication: 18047502] 27. Allergan Botox. Magalhaes E, Menezes C, Cardeal M, Melo A. Botulinum toxic substance kind A versus amitriptyline for the therapy of chronic daily migraine. Clin Neurol Neurosurg. 2010 Jul; 112( 6 ):463466. [Bar, Med: 20399553] 28. Jackson JL, Kuriyama A, Hayashino Y. Botulinum toxic substance A for prophylactic treatment of migraine as well as stress headaches in adults: a meta-analysis.
Allergan Botox - The Facts
Frustration. Onabotulinumtoxin, A for treatment of persistent migraine why not try here headache: pooled analyses of the 56-week PREEMPT clinical program. Migraine.Botulinum toxic substance kind a for the prophylaxis of chronic everyday migraine: subgroup analysis of individuals not getting various other prophylactic medications: a randomized double-blind, placebo-controlled research. Headache. 2005 Apr; 45( 4 ):315324. [Pub, Medication: 15836567] 36. Sandrini G, Perrotta A, Tassorelli C, Torelli P, Brighina F, Sances G, et al. Botulinum toxin type-A in the prophylactic therapy of medication-overuse frustration: a multicenter, double-blind, randomized, placebo-controlled, parallel team study.
[mentioned 2014 Jan 8]; Available from: http://www. ncbi.nlm. nih. gov/pmc/articles/ PMC3139089. [PMC totally free write-up: PMC3139089] [Bar, Medication: 21499747] 37. Ondo WG, Vuong KD, Derman HS. Botulinum toxin A for persistent daily migraine: a randomized, placebo-controlled, identical design research study. Cephalalgia. 2004 Jan; 24( 1 ):6065. [Pub, Med: 14687015] 38. Vo AH, Satori R, Jabbari B, Green J, Killgore WD, Labutta R, et al.
Not known Details About Allergan Botox
Aviat Room Environ Medication. 2007 May; 78(5 Suppl): B113B118. [Bar, Medication: 17547312] 39. Anand KS, Prasad A, Singh MM, Sharma S, Bala K. Botulinum contaminant kind A in prophylactic therapy of migraine headache. Am J Ther. 2006 May; 13( 3 ):183187. [Pub, Med: 16772757] 40. Conway S, Delplanche C, Crowder J, Rothrock J. Botox therapy for refractory chronic migraine.Botulinum toxic substance type A for the prophylactic treatment of persistent daily headache: a randomized, double-blind, placebo-controlled trial. Blumenkron D, Rivera C, Cuevas C. Efficacy of botulinum contaminant type A in clients with migraine. 44.Millan-Guerrero RO, Isais-Millan S, Barreto-Vizcaino S, Rivera-Castano L, Rios-Madariaga C. Subcutaneous histamine versus botulinum contaminant type A in migraine headache treatment: a randomized, double-blind research study.
Cady R, Schreiber C. Botulinum toxin kind A as migraine preventative treatment in individuals previously stopping working oral prophylactic treatment due to conformity concerns. Migraine. Evers S, Vollmer-Haase J, Schwaag S, Rahmann A, Husstedt IW, Frese A. published here Botulinum toxic substance A in the prophylactic treatment of migraine-- a randomized, double-blind, placebo-controlled research.
Facts About Allergan Botox Uncovered
Botulinum contaminant type a for the prophylaxis of chronic everyday headache: subgroup evaluation of individuals not obtaining other prophylactic medications: a randomized double-blind, placebo-controlled research. Frustration. Botulinum contaminant type-A in the prophylactic therapy of medication-overuse headache: a multicenter, double-blind, randomized, placebo-controlled, parallel group study.[pointed out 2014 Jan 8]; Available from: http://www. ncbi.nlm. nih. gov/pmc/articles/ PMC3139089. [PMC totally free post: PMC3139089] [Club, Med: 21499747] 37. Ondo WG, Vuong KD, Derman HS. Botulinum contaminant A for persistent daily headache: a randomized, placebo-controlled, parallel style research study. Cephalalgia. 2004 Jan; 24( 1 ):6065. [Bar, Medication: 14687015] 38. Vo AH, Satori R, Jabbari B, Environment-friendly J, see this here Killgore WD, Labutta R, et al.
Botulinum toxin kind A for the prophylactic treatment of persistent daily headache: a randomized, double-blind, placebo-controlled test. Blumenkron D, Rivera C, Cuevas C. Efficiency of botulinum contaminant kind A in clients with migraine headache. 44.Millan-Guerrero RO, Isais-Millan S, Barreto-Vizcaino S, Rivera-Castano L, Rios-Madariaga C. Subcutaneous histamine versus botulinum toxic substance kind A in migraine headache prophylaxis: a randomized, double-blind study.
Cady R, Schreiber C. Botulinum toxic substance kind A as migraine headache precautionary therapy in clients formerly stopping working oral prophylactic treatment due to conformity problems. Headache. Evers S, Vollmer-Haase J, Schwaag S, Rahmann A, Husstedt IW, Frese A. Botulinum contaminant A in the prophylactic therapy of migraine headache-- a randomized, double-blind, placebo-controlled research study.
Report this wiki page